I recently received a question about the potential for humans to convert fat into carbohydrates and thought it was interesting enough to put here on the site, particularly since I’ve been talking about ketosis so much recently (both on ClimbSci with Tom and then in a subsequent article). At any rate, it gives me another opportunity to put a picture of the citric acid cycle in an article, so there’s that too, haha.
Most people know that we can turn carbohydrates into fat and understand the role of such a pathway (that is, it allows us to store calories in a long-term fashion regardless of where they come from). It’s disputable how much this actually happens,* but it’s indisputable that the pathway exists.
What about the other way around, though? Again, I think most people know that it doesn’t happen—biochemical workarounds like ketogenesis would be completely unnecessary (not to mention likely non-existent) if we could easily turn fats into carbs—but is that the whole story, or is there a chapter missing? Let’s take a look.
Revisiting Endogenous Glucose Production
Before we get to the question of fat-to-glucose conversion, let’s look at the whole of endogenous glucose production—that is, gluconeogenesis. Gluconeogenesis is fed by a few molecules, which we’ll go over in turn.
The most frequent fuel for gluconeogenesis is lactate, which is produced during anaerobic exercise. In anaerobic exercise, glycolysis splits a glucose molecule into two pyruvates, but those pyruvates can’t enter the citric acid cycle without oxygen so they’re reduced to lactates. Glycolysis yields a net two ATP (four total, but it costs two), leaving about 34 ATP remaining in the lactate molecule. If our body couldn’t take that lactate molecule and turn it back into glucose, we’d lose out on a lot of energy—thus, gluconeogenesis. You might correctly note, however, that there’s no real net gain of glucose here; we’re just turning a molecule that once was glucose back into glucose.
We can also convert glycerol (the sugary backbone of a triglyceride molecule) into glucose. This is truly “new” glucose creation—it comes from our diet in a non-glucose form—but it doesn’t account for much when all is said and done. The glycerol part makes up only about 10% of the mass of most common triglycerides, and calorically accounts for only about 1 calorie out of every 40-50. If you consumed 100 grams of fat in a day, you might get around 40 carb calories out of it (but probably less when the cost of gluconeogenesis is factored in).
If we actually want to produce a decent amount of “new” glucose, then we have only a single source to turn to: protein. Or, to be more specific, amino acids. Amino acids are classified as either “glucogenic”—meaning they can be converted into glucose—or “ketogenic”, meaning they cannot. Whether an amino acid is one or the other (some are both) depends on where they enter the citric acid cycle:
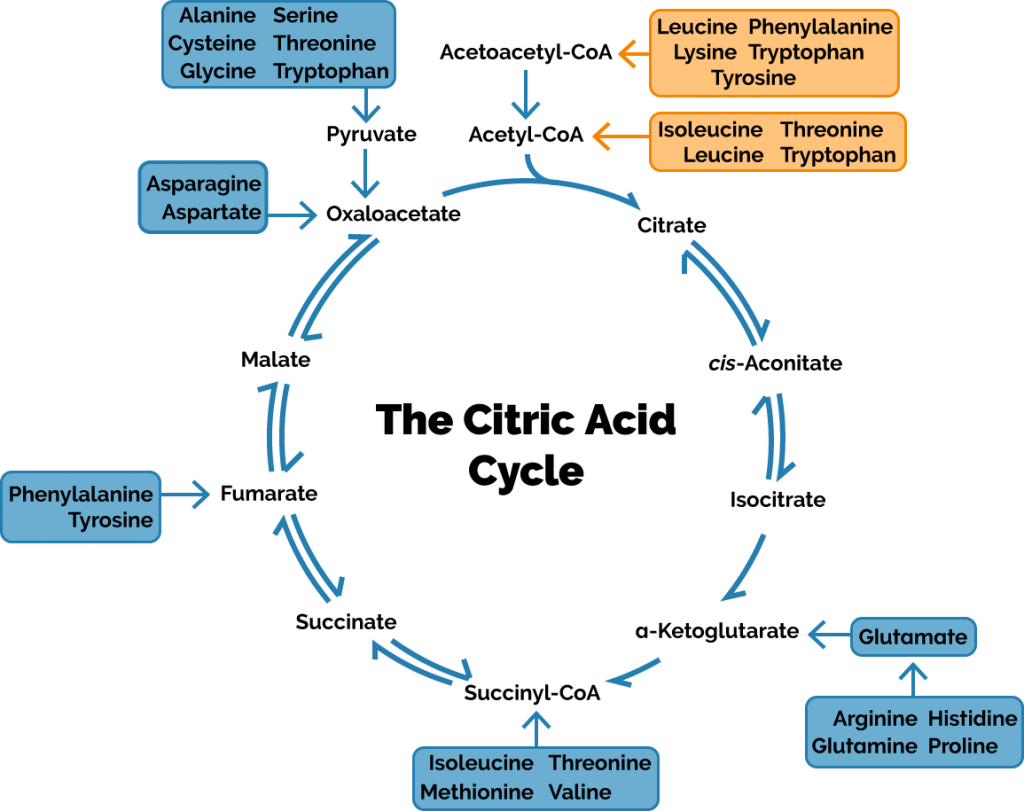
If the above graphic seems really confusing, don’t worry, there’s only a single important thing to pay attention to: whether an amino acid enters the cycle directly or as acetyl-CoA. If an amino acid can enter directly, it will wind its away along and eventually become a new, never-before-seen molecule of oxaloacetate. If it has to enter as acetyl-CoA, then it requires an input of oxaloacetate and there can be no oxaloacetate profit. Oxaloacetate is the molecule that gets fed into the gluconeogenesis pathway, so if you can’t churn an oxaloacetate profit, you can’t make a glucose profit, either.
The amount that protein contributes to gluconeogenesis depends on your diet and circumstances. For most of us—humans that are not starving and who eat a mixed diet—gluconeogenesis contributes relatively little. It contributes a bit here and there as necessary, but doesn’t make much of an impact on our daily glucose “load”. If you’re starving (as in literal, life-is-at-stake hungry), then it’s likely contributing a lot as your muscles are broken down and converted to sugar to keep your brain alive. In ketosis, it’ll be somewhere in-between (but closer to the “normal diet” end of the spectrum than starvation).
To recap, the only molecules our body can really use to profitably create glucose are the glucogenic amino acids. The amount of glucose we derive from the glycerol in triglycerides is negligible, and the majority of lactate we convert into glucose began its life as glucose, so there’s no profit there.
Why Not Fat, Though?
Like the ketogenic amino acids, fat can only enter the citric acid cycle through acetyl-CoA. Acetyl-CoA requires a molecule of oxaloacetate to enter the citric acid cycle which means there’s no net gain in molecules of oxaloacetate.
Some organisms—actually, basically every organism other than us animals—have a different cycle (the glyoxylate cycle) in which they can effectively convert acetyl-CoA into new molecules of oxaloacetate with which to create sugars. For non-animal organisms, this is critical to survival because their cells have carbohydrate-based cell walls;** no cell walls, no life.
That might sound special, but it’s not; it’s mostly because these organisms are non-motile and thus must be able to synthesize every organic molecule they may need. In addition to being able to synthesize carbohydrates, these organisms can also synthesis folate (vitamin B9), beta-carotene (vitamin A), ascorbic acid (vitamin C), and at least ten other organic molecules we humans must obtain from our diet.*** We don’t have the pathway because we don’t need it.
A Possible Exception: Acetone
The question I referred to at the beginning of this article linked to an article describing a role for acetone in gluconeogenesis. The article is paywalled, but it describes two pathways through which our body could potentially convert acetone into glucose: the methylglyoxal pathway and the propanediol pathway. Both pathways convert acetone into lactate, which can then enter gluconeogenesis.
In a technical sense, then, fat could be converted to glucose by a human—but it’s a long and convoluted path that requires the input of an uncommon metabolic byproduct of ketosis (acetone) and meanders through a molecule with known cellular toxicity (methylglyoxal) to reach its goal. For animals, this pathway seems to act more as a safety valve that just happens to vent into glucose rather than a legitimate pathway for new glucose creation.
Beyond the exploration of the methylglyoxal and propanediol pathways seen in the article above, we really haven’t examined the biochemical fate of acetone, or at least the acetone we don’t exhale (as most of it is). One article found increased levels of methylglyoxal in ketotic patients, suggesting a possible upregulation of the methylglyoxal pathway—but methylglyoxal can also be formed in numerous other ways, so how much acetone is actually converted to glucose is unclear (though it’s likely more than the “zero” my biochemistry textbook describes).
Ultimately, I would dare say it doesn’t really matter except in a pedantic sense. We’re really not making very much acetone to begin with—even in ketosis—and the small amount of acetone we do form would be a comparable drop in the ocean of gluconeogenic precursors even if it was 100% converted to lactate. The pathway leading from acetyl-CoA to glucose through acetone is incredibly energetically disadvantageous (we lose 25% of the acetyl-CoA molecule when acetoacetate is decarboxylated to acetone), and only really makes sense from the perspective of detoxification. It’s an interesting example of a biochemical workaround, but from a gluconeogenic standpoint it’s trivial.
Fats Are Fats to Stay
When we look at all the trouble our body goes to with ketosis just to keep the brain fed and happy in a state of dietary carb insufficiency, it might seem strange that our body lost the ability to convert fats to carbohydrates. At the end of the day, though, we can only assume that such a pathway was unnecessary for animals, who generally either consume carbohydrates as part of their daily diet (herbivores and omnivores) or who have exceptional enzymatic machinery to convert amino acids into glucose (carnivores).
Regardless of why we can’t convert fats to carbs, it’s safe to continue saying that no pathway really exists, with an addendum that we probably can convert some acetone derived from ketogenesis (derived from fats) into glucose. Who knows, maybe it’ll earn you some points in a trivia night!
Notes
* There’s no question that consuming excessive carbs as a part of a calorically excessive diet will cause fat gain. In most circumstances, this fat gain is not the result of de novo lipogenesis, however, but rather due to the shunting of caloric expenditure to carbohydrates and away from fats. Thus, in a calorically excessive carb-heavy diet, our body preferentially burns carbohydrates for energy and stores the excess energy with dietary fats. Biologically this makes total sense since converting molecules is costly and there’s no reason to pay that cost when we already have an equivalent molecule (dietary fat) available.
** Many of you might be thinking, “but plants create sugars during photosynthesis!”, and that’s true. The critical part comes before a plant can photosynthesize, though—it comes during seeding. Seeds are calorically dense because they’re rich in fat, and they’re rich in fat not to please our palates but to provide sufficient energy to a seedling so it can “make it” until photosynthesis can commence. If the plant couldn’t turn those fats back into sugars, it couldn’t synthesize cell walls and would quickly die.
*** We also require many non-organic molecules—iron, calcium, magnesium, etc.—but so do plants, fungi, bacteria, archaea, and protozoa. They rely on the environment to provide those components, and are limited in the environments they live in by the elements they require. Evolutionary adaptation allows them to work around those limitations.














very good article. however, it appears that herbivores already do this. convert acetone into glucose. their diet is rich in acetone and other short-chain fatty acids.
I’m talking only about human physiology because every living thing has evolved different strategies for existence. Koalas eat only eucalyptus leaves and can do so because their physiology has the biochemical pathways to make any nutrients they need that eucalyptus lacks. Humans, unfortunately, lack a reliable pathway for the conversion of fats to carbs.