I’ve written before about how carbohydrates are more oxygen-efficient than fat, which is a boon for climbing of all types; what I haven’t really addressed is why. Far from being a dry, biochemical fact of interest only to physiology enthusiasts, knowing exactly how carbohydrates derive their advantage can inform everyone about their diets, as well as help explain why the majority of climbers do not benefit from a low-carb diet performance-wise. Truly, it’s all in the structure!
Don’t worry if chemistry isn’t your strongest suit, I’ll do my best with descriptions and pictures to demonstrate how carbohydrates have an inborn oxygen advantage.
The Basic Structure of Carbohydrates and Fats
Nutritionally and biologically speaking, there are a huge number of unique carbohydrates and fats. Structurally, however, all carbohydrates resemble one another, as do all fats.
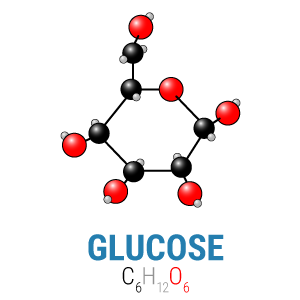 Within the carbohydrate group, different types of chains made from different types of simple sugars create numerous carbohydrate-based macromolecules, from simple sucrose (table sugar, a glucose linked to a fructose) to amylose (starch, thousands of glucose molecules connected by links our bodies can digest) to cellulose (wood fiber, thousands of glucose molecules connected by links our bodies cannot digest). Despite their diversity, however, all these molecules are formed of simple sugars, and all simple sugars have the same basic formula: Cn(H2O)n. That means that every carbohydrate has exactly one carbon and oxygen atom for every two hydrogen atoms—or that carbon and oxygen are always found in equal measure in any carbohydrate.
Within the carbohydrate group, different types of chains made from different types of simple sugars create numerous carbohydrate-based macromolecules, from simple sucrose (table sugar, a glucose linked to a fructose) to amylose (starch, thousands of glucose molecules connected by links our bodies can digest) to cellulose (wood fiber, thousands of glucose molecules connected by links our bodies cannot digest). Despite their diversity, however, all these molecules are formed of simple sugars, and all simple sugars have the same basic formula: Cn(H2O)n. That means that every carbohydrate has exactly one carbon and oxygen atom for every two hydrogen atoms—or that carbon and oxygen are always found in equal measure in any carbohydrate.
In the picture to the right, you can see how the carbons (the black balls) equal the oxygens (the red balls) in number. This is important for a reason we’ll come back to in a bit.
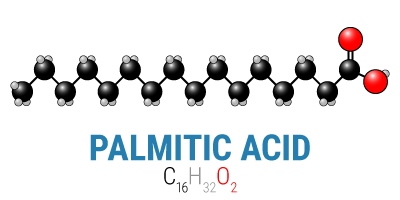
Fats, too, have tremendous diversity. There are saturated fats, polyunsaturated fats, and monounsaturated fats, and each of these broad categories has numerous chain lengths ranging from only two carbons long to over thirty carbons long. And as with carbohydrates, all fats have the same basic structure with only a small amount of variation: CH3(CH2)nCOOH.* In reality, however, only the tail end—the “CH3(CH2)n” part—is important as a fuel, which means is that for all intents and purposes fats do not contain oxygen, and the body must provide all the necessary oxygen itself.
 *[Please note, this is only for saturated fats, or fats that are fully saturated with hydrogen atoms. When fats are unsaturated to some degree, they have fewer hydrogen atoms and therefore a slightly different chemical structure, but one that still only contains oxygen in the “COOH” (carboxyl) end. For the purpose of this discussion on fuels, oxygen, and fat metabolism, there’s no important difference.]
*[Please note, this is only for saturated fats, or fats that are fully saturated with hydrogen atoms. When fats are unsaturated to some degree, they have fewer hydrogen atoms and therefore a slightly different chemical structure, but one that still only contains oxygen in the “COOH” (carboxyl) end. For the purpose of this discussion on fuels, oxygen, and fat metabolism, there’s no important difference.]
Ultimately, the body will break either of these fuels down into carbon dioxide (CO2), yielding energy in the process. In order to be able to break them down, however, it’s necessary to balance the input (the carbons and oxygens from carbohydrates or fat) with the output (carbon dioxide). When you start with a glucose molecule and its six carbons and six oxygens, you only need to add an additional six oxygens to balance the equation. On the other hand, when you start with a 16-carbon fat like palmitic acid above, you need to add thirty two oxygens, or one for each carbon that will go through the citric acid cycle (the means by which we aerobically metabolize energy).
Basically, all carbohydrates come equipped with oxygen in equal measure, which means it takes less oxygen to aerobically metabolize them. Fats don’t come equipped with oxygen, so you need to provide more to get the same benefit.
To sum up:
- Carbohydrates come equipped with one oxygen for every carbon, and only need one additional oxygen per carbon to metabolize aerobically.
- Fats do not contain oxygen (in the fuel-part of the molecule), and need two additional oxygens per carbon to metabolize aerobically.
When oxygen is plentiful—which is most of the time—that small advantage of carbohydrates over fats is meaningless. Oxygen is free, after all, and our body literally harvests it without needing to think about it. But during intense exercise, or any time your muscles are deprived of oxygen for any reason, then carbohydrates gain a distinct and incontrovertible oxygen advantage, estimated to be roughly 20% (as in you get 20% more energy per unit of oxygen when using carbohydrates as a fuel than when using fat as a fuel).
Oxygen Is at a Premium for Climbers
Okay, but climbing isn’t really the sort of sport that makes you breathe heavy in a desperate attempt to perfuse the blood with oxygen. You may find your heart beating faster by the end of a climb, but you rarely finish completely out of breath as you might during a run or swim. Is oxygen really at a premium for climbers?
Yes! Oxygen is a limited commodity for climbers—not because we can’t inhale enough, however, but because we can’t deliver enough.
Unlike most other sports, climbing relies heavily on isometric contractions—muscle contractions that neither shorten nor lengthen the muscle. While other sports have rapid muscle contract-relax cyles, where the muscle gets to relax at regular intervals, climbers often spend long chunks of time contracting their muscles before allowing them to rest. As a result, blood flow is occluded to the working muscles—and without blood, you have no oxygen.
Absolute occlusion is estimated to occur at about 65% of maximal isometric contraction for most people, but could be as low as 51% for stronger individuals (because their maximal strength is so much higher, and because blood vessels don’t become more resistant to contractile pressure as you get stronger, the overall percentage is lower even if the absolute force required to occlude vessels remains the same). This occlusion leads to an oxygen deficit, which drives greater reliance on anaerobic energy systems that ultimately drive down intramuscular pH and lead to fatigue—so even if it’s impractical to know exactly what force occludes your blood vessels, it suffices to say that you’ve experienced this occlusion.
That means that anytime you’re gripping onto something, particularly for more than a few seconds, you’re depriving your muscles of oxygen. The far greater consequence of this is the shift towards anaerobic fuel metabolism and the concurrent build-up of free hydrogen ions in the muscles (the cause of intramuscular acidosis, and one suspected culprit in fatigue)—but with sufficient carbohydrates, you can at least eke out a bit more aerobic energy from the remaining oxygen than you could with fat.
The Take Home Message
So how does all this play into your climbing? Let’s recap fast:
- Carbohydrates require less oxygen to metabolize aerobically than fat, giving carbohydrates a per-unit-of-oxygen energy bonus.
- Climbers rely heavily on isometric contractions that occlude blood flow, and therefore oxygen supply, to the working muscles.
- Without sufficient oxygen, the muscles must rely on anaerobic energy production, a process that rapidly increases intramuscular acidity.
- Since carbohydrates can produce more aerobic energy with less oxygen, they can make the limited supply of oxygen in a contracted muscle go further, helping prevent fatigue.
In other words, carbohydrates hold an advantage over fat in terms in of energy production during climbing because carbohydrates don’t need nearly as much oxygen to metabolize. It’s a small advantage considering how reliant climbing is on anaerobic energy sources regardless (because anaerobic energy sources are capable of far more energy output per second), but it’s an advantage worth taking nonetheless!
A Word from Our Sponsors
Actually, Climbing Nutrition doesn’t have any sponsors. We don’t run ads, sell products, or do anything else that might bias the quality and content of the information we share. Instead, we run off monthly contributions from you, our readers!
Climbing Nutrition is, always has been, and always will be a free resource. Everything we offer, no matter how in-depth, will always be free—not the freemium model that so many sites use, where they give some basic information for free in the hopes you’ll pay for the real stuff. That’s just not our vibe.
If you want to join the list of Climbing Nutrition’s illustrious patrons and help support high-quality, scientifically accurate, free access climbing-specific nutrition information (and we hope you do!), you can do so here. It’s quick, easy, and pain-free—and you can rest easy knowing you’re helping this resource grow and improve.
Thanks!
Brian















Would really like to have a printer friendly version
Loved the info; never knew where that 20% extra oxygen number came from
Thanks, Fred
I completely agree with all of your conclusions in this article about the oxygen-efficiency of carbohydrate metabolism versus fats but the chemical justification you presented is a bit off.
From a thermodynamic stand point fat is a better fuel and you should be able to get more work out of burning a “fat carbon” versus a “glucose carbon”. To use the glucose/palmitic acid example in the article glucose has a heat of combustion of 670 kCal/mol and palmitic acid has a value of 2400 kCal/mol. Palmitic acid has 16 carbons and glucose has 6 carbons. Therefore, palmitic acid yields 150 kCal/mol carbon compared to 112 kCal/mol carbon in glucose. The math is the same if you calculate the energy on a carbon dioxide produced basis as you attempted to do in the article. That said the glucose gives you less energy per CO2 produced contrary to your point. This intuitively makes sense too since the glucose carbon is already partially oxidized as you pointed out in the article. The heat/work produced from the chemical reaction is from oxidation, therefore, the more oxidized the fuel is the less heat/work you can get out of it.
Now the fact is that the body’s metabolism is far from 100% efficient just like how your car’s engine only converts about 25-50% of the energy in the gasoline into motive power. The rest of the energy from combustion goes out the tailpipe as hot gas. What is interesting that is presented in this article (and others) is that your body’s “engines” also have different efficiencies for the various metabolic oxidation pathways. If you look at the useful energy generated from oxidation of fuel (i.e. production of ATP) you can start to compare the pathways on an oxygen efficiency basis. As shown in your article “ An In-Depth Look at Energy Metabolism: Part I” for every mol of glucose you aerobically metabolize you yield 38 mol ATP using a total of 6 mol of oxygen. For palmitic acid you yield 131 mol ATP per 23 mol oxygen. So comparing on an oxygen efficiency basis you get 6.3 mol ATP/mol O2 and 5.7 mol ATP/mol O2 for glucose and palmitic acid, respectively. So aerobic glucose metabolism is better! But it is because of your body’s metabolic efficiency differences not because of the inherent energy in the fuel.
I’m not a biochemist (I’m a chemical engineer) so I started to wonder where the “extra” energy is going. Because of the conservation of mass and energy the “extra” inherent energy you get from burning the fat has to go somewhere. The oxidation could either be indirectly chemically coupled to other metabolic reactions or lost in the form of heat. I know I generate a lot of heat since I get pretty sweaty when I aerobically exercise!
The actual “how” of metabolism is way more complex than what is presented here and it would be really interesting to learn why carbohydrate aerobic metabolism it is more efficient than fat metabolism. Maybe it has something to do with how we evolved. I wonder if mammals that evolved in cold climates have less efficient metabolisms so they generate more heat.
All that said I think I’ll hedge my bets and eat some ice cream before trying to send my next project.
I suspect any “extra” energy will go into regenerating creatine to creatine phosphate, not heat production. From the perspective of exercise, heat production is more than a “waste” of energy or a loss of efficiency—it’s a harmful side effect that ultimately limits and endangers us (due to overheating). Since we’re not 100% efficient creatures, there will always be loss of energy as heat and subsequent increases in body temperature, but when extra energy exists heat production would be a last resort unless body temperature is below normal. There should only ever be a small amount of “extra” energy, though, since ATP turnover is constant (especially during exercise) and so there will always be a target in the form of AMP, ADP, or creatine.
All that being said, dietary fat—say in the form of ice cream!—still suffers from other biological limitations. For example, we have a limited number of fat transport proteins that we share between digested and absorbed dietary fat and the fatty acids released from adipose cell lipolysis. During exercise, these proteins become saturated regardless of recent food intake and our body begins to rely more heavily on intramuscular fat stores which do not require transport proteins. Glucose, on the other hand, does not require blood transport proteins, and can therefore be increased through dietary means before exercise without limiting or inhibiting the use of other sources of glucose.
At any rate, hedge away and have some ice cream before your next project. At the very least, the sugar in it will increase performance and the fat is unlikely to have a negative impact (unless you’re eating pints at a time) so there’s no downside!
It’s not just about the efficiency of your body at converting glucose vs fat to energy. Glucose has an advantage even when burned outside of your body (say, in a bonfire). Consider that carbs deliver four calories per gram to fat’s nine calories per gram. Now one gram of glucose requires 1.07 grams of oxygen, giving you 3.74 calories for each gram of oxygen. On the other hand, burning one gram of palmitic acid requires 2.875g of oxygen, or only 3.13 calories per gram of oxygen.
In a way, it’s similar to comparing the use of alcohol vs gasoline in a racecar. A gallon of gas has more energy than a gallon of ethanol. But ethanol needs less oxygen to deliver a given amount of energy. Getting more fuel to an engine is easy – slap on a bigger injector. But more air is VERY difficult in a naturally aspirated engine. Thus ethanol can make more power.
Exactly! Since “air” is consumed so passively—we don’t prepare it or measure it, and we consume it unconsciously the majority of the time—we tend to underplay its role in combustion. Yet our body is not an open environment where O2 is an unlimited reagent; we have an incredibly complicated system designed to deliver O2 where it needs to go, and that delivery system winds up being the bottleneck in physical activity sometimes. Thus, reducing our need for O2 reduces the impact of the bottleneck and power output improves.
What I would like for someone to explain to me, from a chemical standpoint, how does your body convert carbs into fat. Because apparently everybody thinks that it does. I’m very much under the impression that it doesn’t, and the extra carbs are pissed or pooped out. And all the food they say you shouldn’t eat because it contains ‘bad’ carbs, actually contain a lot of fat, that is, you guess it, stored as fat in your body.
Can someone explain to me, where all the oxygen goes from this conversion?
The oxygen would most likely find its way into either a molecule of CO2 or H20. The process of converting carbs to fat is well-understood (but not particularly suitable for posting in a comment!), so it’s not really questionable that it happens—the question is “how much?”. In other articles and in ClimbSci, I’ve discussed how most of the time in a state of caloric excess, our body doesn’t convert carbs to fat but rather burns the carbs for energy and stores whatever fat we’ve gotten from our diet (because it requires energy to convert carbs to fat). To induce measurable levels of de-novo fat synthesis, you need to have a diet that is so shifted towards carbs that we have no choice but to convert and store them, but for most people this isn’t the case. Nonetheless, the effect is the same: if you overconsume calories, your body will store some of those calories (regardless of the source) as fat.
This seems to be based on a fallacy. The oxygen inherent in carbs is already spoken for – glucose at C6H12O6 is effectively 6 carbons and 6 water molecules C6-6(H2O), hence “carbo-hydrate”. All of the oxygen to react the carbon has to be supplied, that in the water can’t help. This is why the RER of carbohydrate is 1.0 – 1 molecule of O2 used yields one molecule of CO2. Fats on the other hand have an RER around 0.7 as some of the oxygen is used to oxidise the hydrogens that don’t come with their own oxygen supply.
There may or may not be a favourable outcome in terms of energy out per O2 in, but it doesn’t depend on the oxygen present in the “wet carbon” that is carbohydrate.
There’s no denying the fats are more efficient energy-wise, but if you measure the O2 input and CO2 output of both a fat and a carbohydrate and balance them to be equal in terms of CO2, you can see that oxidizing fat requires more O2:
Oleic Acid: 2C18H34>/sub>O2 + 51O2 –> 36CO2 + 34H2O
Glucose: 6C6H12O6 + 36O2 –> 36CO2 + 36H2O
The RER (VCO2/VO2) shows that fats produce less carbon dioxide per oxygen consumed, so if we balance for carbon dioxide then whichever fuel consumes more oxygen will have the lower RER. It’s not a huge effect, and we’re all burning fat during our aerobic exercise regardless. But, carbs do have a slight advantage when it comes to oxygen-sparing for aerobic activity.
Your comparison would be valid if muscles would burn carbs and fats–but they do not. Muscles burn glycogen (high carb diet) or ketones (low carb diet). The build-up of ketones (which happens during rest and recovery) may require more O2 than the build-up of glycogen, but the O2 requirements for burning of ketones as fuel during exercise is lower than for glycogen. And this is not just chemistry theory–we have tested athletes in and out of ketosis (measuring ketones via breath analyzers an blood) and discovered that VO2Max improves in ketosis.
Ketones aren’t burned directly for energy, they must be converted back into acetyl-CoA which can then be run through the citric acid cycle, yielding ATP both directly and (much more importantly) indirectly through oxidative phosphorylation in the electron transport chain. Regardless of the fuel source—whether carbs, fats, or ketones—it’s acetyl-CoA that is the ultimate carrier of the energy that is yielded from the citric acid cycle and electron transport chain, and thus any oxygen benefit of one fuel or another must happen before this step.
Ketones are derived from fats (they result from a coenzyme A-related bottleneck) and do not have any oxygen advantage compared to fats because they’re literally byproducts of fat metabolism but with a few more steps thrown in. None of these steps introduce oxygen from another source to improve the oxygen efficiency of the fuel, so ketones are biochemically still less energy efficient than carbohydrates. When we start looking at big picture effects like VO2max we cannot disentangle all the other things going on both diet- and exercise-wise, and so if we see improvements in VO2max during a ketogenic diet we must assume it comes from a source other than the biochemical oxygen efficiency of carbs vs. fats. From my own study of the topic, however, I’ve yet to see a paper clearly demonstrate a performance advantage to a ketogenic diet, even if some parameters (like VO2max) occasionally improve. In the end, it’s total performance that matters more than isolated variables!